I am one of those lucky people who gets to do what I love; I live, work and literally breathe my research. I am also one of those very lucky people who do not suffer from hayfever; which is lucky, as after a day phenotyping my oilseed rape I am literally covered in yellow specks of pollen (Image 1). I am a plant scientist, and my research ranges from looking at the tiny molecular changes of seeing what a single gene and protein does, to studying the physiology in 100s of lines. As you can probably tell by now, I am interested in pollen development and everything related to it: the male reproduction organ (the anther), the environment, plant hormones, and how everything works together to produce these amazing little packages which allow seed to be produced.

I am currently a Post-Doc researcher in the BBSRC BRAVO (Brassica Rapeseed and Vegetable Optimisation) project at the University of Nottingham, United Kingdom. This is an amazing project looking at all aspects of Brassica (oilseed rape, mustard, kale etc) development from seeds, germination, flowering timing, flowers (and importantly pollen) back to yield and seeds again. BRAVO uses the natural variation within Brassica species to understand the genes networks controlling vital processes and relationships between genes to target crop improvement. This project brings together UK plant scientists and industries to increase robustness in crop performance, and adaptation to environmental change. I am lucky to be part of this fantastic and supportive team.


So what do I actually do, day-to-day? As I mentioned, I look at physiology in big trials of 100s of different lines to look at natural variation. Image 2 shows the start of my current trial – they will grow over the winter similar to in fields across the country, and will start to flower in spring. When spring comes around, I have a LOT of microscope work (Image 3), and I am excited about the results that I am going to see. Using confocal microscopes, I can look at pollen and anthers in spectacular detail. I will be looking at pollen viability and pollen germination to see which lines produce the healthiest pollen and which lines produce the poorest pollen. I will then compare the differences between these lines to see what genes could be involved.
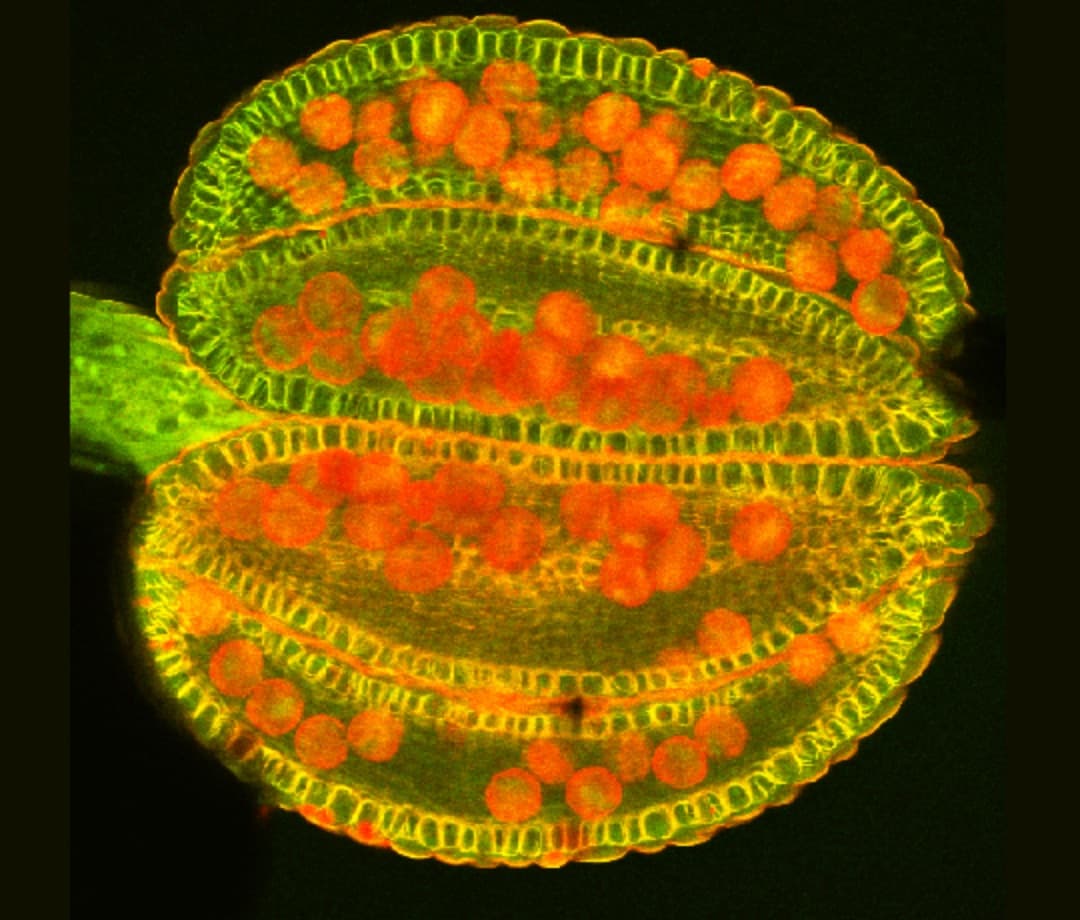
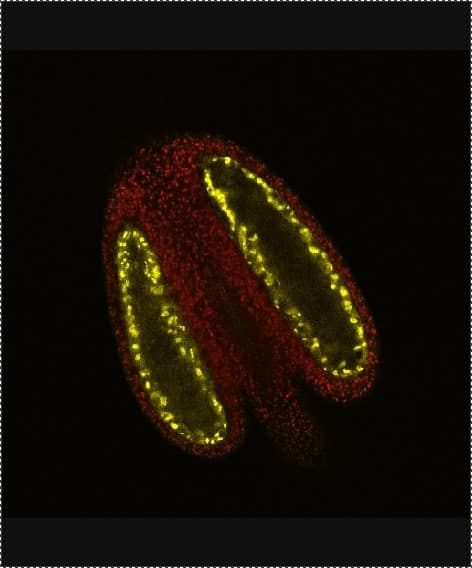
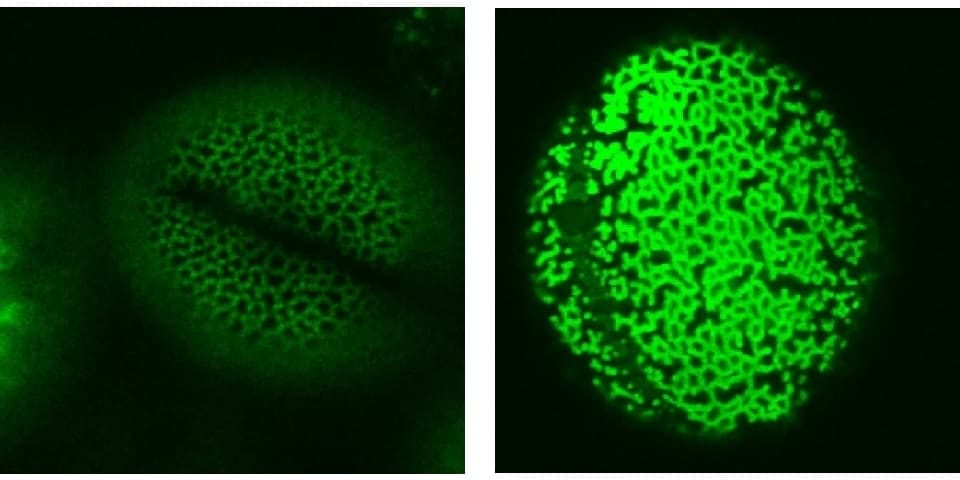
Using microscopy I can also study different aspects of pollen development in more detail. Using different stains in Brassica and Arabidopsis (a model plant) I can highlight different aspects of the anthers and pollen. Image 4 shows the lignin cell wall deposits in the endothecium tissue (yellow) that is essential for pollen (red) release into the environment. As well as stains, we also use reporter fluorescent tags, which are linked to proteins of interest to find out exactly where and when they are expressed. Image 5 shows the aborted microspore protein linked to yellow fluorescent protein, which is expressed in the nucleus of the tapetum tissue within the anther, and is required for pollen development as published in New Phytologist. Using tags such as these gives us increased understanding of where the proteins are, and what they are doing. To further this knowledge, we also use mutant plants where the protein of interest is no longer functional, to see what effect it would have on the plant. In image 6 and 7 we use a stain to highlight the pollen wall – in particular part of the wall made up of exine – we can see that exine patterning on the pollen is different in the wildtype (image 6) and a mutant (image 7). In the mutant, the regular pattern is now broken and incomplete in places.
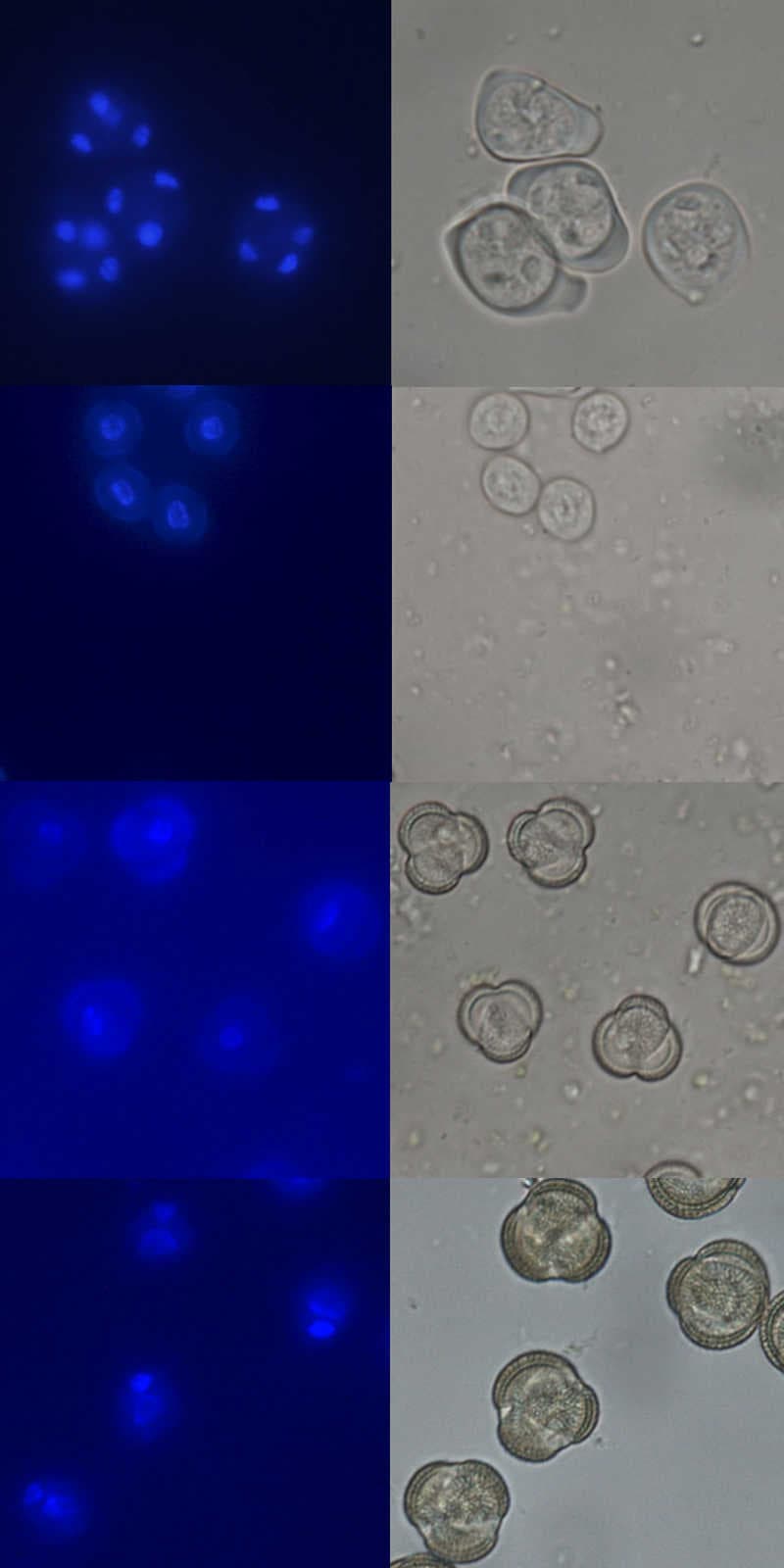
From my research I can look at differences (phenotype) caused by a missing protein (mutant), where the protein is normally expressed such as in exine staining above. But it is also important to know exactly when the proteins are expressed in development. To do this, we use a stain to label the nucleus of pollen going through meiosis, mitosis I and mitosis II to produce mature pollen. The stain allows us to determine the exact developmental stage. Image 8, 9, 10 and 11 show the nucleus stained in blue on the left and the light image on the right. By looking at the nucleus number and position and pollen size, shape and pollen wall, we can tell what stage they are at.
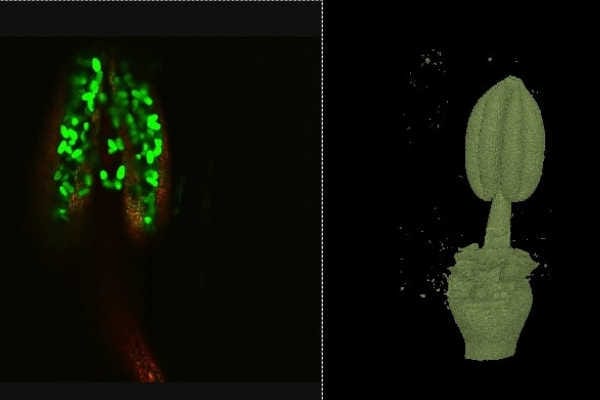
We are also lucky in Plant Sciences to have a light sheet microscope and micro X-ray CT-scanner. These produce images in 3D, allowing me to have more understanding of the size and shape of my samples, and how the pollen is positioned within the anther (image 12 and 13 respectively). The X-ray CT scanner also has the benefit that we can look inside the anthers without dissecting them, and in the case of Barley, look at the developing flowers within the stem (for more details see my work published in Plant Methods)
Using the tool of microscopy I can discover a wealth of information about anthers and pollen, as well as the proteins that play a role in pollen development. They help me to reveal the secrets of pollen, and the importance it plays in producing food.




