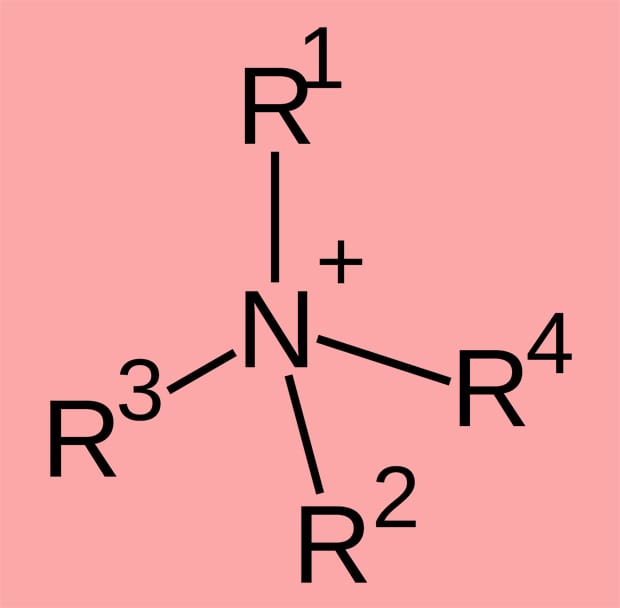
Nitrogen is an essential macronutrient for plants – i.e. they cannot complete their life cycle without it – and it is needed in relatively large amounts. Although it is abundant in the atmosphere – N2 (‘dinitrogen’) comprises about 80% of the Earth’s invisible gaseous envelope mantle – that molecule cannot be used directly by plants. Instead they rely on oxidation of nitrogen to NO3– – ‘nitrate’ – since that is the form in which most plants absorb the nitrogen they need from the soil. Although some imaginative plants, like legumes, can supplement their nitrogen intake using NH3 produced by symbiotic microbes (that ‘fix’ N2 directly from the atmosphere), I thought that such inorganic N sources were about it as far as root-routed plant N-sources went. Astonishing news then that ‘quaternary ammonium compounds [think inorganic ammonium – NH4+ – but with organic groups replacing each of the four hydrogens] can be abundant in some soils and are taken up as intact molecules by plants’, because these are organic N-compounds.
Charles Warren demonstrates that ‘two ecologically disparate species’ (an understatement if ever there was!) – non-mycorrhizal Banksia oblongifolia and mycorrhizal Triticum aestivum (wheat) – take up intact molecules of betaine, carnitine and acetyl-carnitine. Two key findings of the study are that ‘the pool of small, nonpeptide organic-N in the soil solution is chemically diverse and not dominated solely by protein amino acids’, and that plants have an ‘even broader palate than is suggested by most of the literature on organic N’(!). I’m grateful to that article for putting me straight on the fact that other soil-sited organic N-sources – such as intact amino acids – can be used by plants as well. All of which suggests one has to be very careful in assessing the N-status of soil as a suitable growing medium for plants – have all possible plant-usable N-sources been considered and quantified? Maybe N is not in such short supply as frequently stated…? Maybe we don’t need to add as much expensive N-fertiliser to achieve decent crop yields as purveyors of N-fertiliser might like us to believe…? Certainly, time to update those plant mineral nutrition lecture notes (again…).